can uv containing compounds be analyzed under gas chromotography|gas chromatography uv spectroscopy : Brand manufacturer The purpose of this article is to provide an overview of the features and specificities of gas chromatography–vacuum ultraviolet spectroscopy coupling which has gained interest since the recent introduction of a commercial vacuum ultraviolet detector. webProvavelmente por conter alguma palavra proibida. Grupo. 👤 4.292. 🔞 Modelos Verificadas. S. 😈TUDO LIBERADO 😈 (sem regras)🔞 - link do grupo no telegram.
{plog:ftitle_list}
WEB5 de jun. de 2022 · A link to download R-User Games and yPER Studios’ free-to-play Spider-Man: Miles Morales Android can be found in the comments section of the trailer .
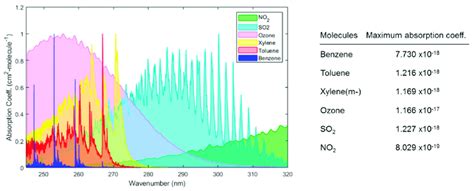
The purpose of this article is to provide an overview of the features and specificities of gas chromatography–vacuum ultraviolet spectroscopy coupling which has gained interest since the recent introduction of a commercial vacuum ultraviolet detector. Using UV spectroscopy as a selective detection approach in gas chromatography (GC) has been demonstrated here with a diode array detector (DAD). The benefits of using an UV detector in GC were realized through .ionality of Gas Chromatography are important to get reliable results. For GC analysis, compounds containing functional groups with active hydrogens such as -SH, -OH, -NH and -COOH are of . Gas chromatography is widely used for the analysis of a diverse array of samples in environmental, clinical, pharmaceutical, biochemical, forensic, food science and .
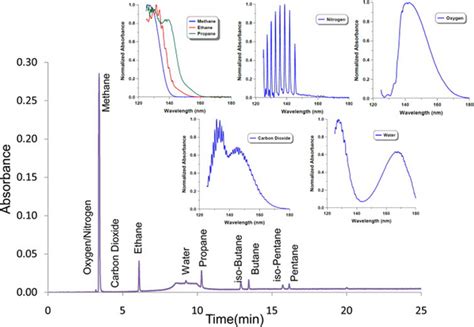
Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. The stationary phase is either a solid adsorbant, termed gas-solid chromatography . The vacuum ultraviolet spectrophotometer was developed recently as an alternative to existing gas chromatography detectors. This detector measures the absorption of gas-phase chemical species in the range of .
A new gas chromatography detector based on vacuum ultraviolet (VUV) spectroscopy (GC–VUV), which simultaneously collects full scan (115–240 nm) VUV and UV .VUV spectroscopy is a relatively new GC detection methodology that combines qualitative spectral identification - similar to mass spectrometry - with faster flow rates, allowing for shorter run times while still maintaining high accuracy and .
Axial Deviation Tester supplier
VUV spectra can also be used to deconvolve analyte coelution, resulting in an accurate quantitative representation of individual analyte contribution to the original response. 2 This characteristic lends itself to . As stated earlier, TLC can be used to ensure purity of a compound. It is very easy to check the purity using a UV-light. The identification of most compounds can be done simply by checking \( R_f\) literature values. . Chromatography is regarded as one of the most significant bioanalytical techniques used in different branches of life sciences and chemistry. It allows the separation, identification, and purification of the compounds of diverse origin, class, and nature from a complex mixture qualitatively and quantitatively. Introduction to gas chromatography Gas chromatography is a chromatography technique that can separate and analyze volatile compounds in gas phase. Depending on stationary phase used in this analytical .
Chromatography is an analytical technique used to separate a given mixture into its components. The technique is based on the principle that when a mixture and a mobile phase are allowed to flow over a stationary phase, the separation occurs based on the differential affinities of the components for these 2 phases.[1] Chromatography is a commonly used .Analysis time can vary from several minutes for samples containing only a few constituents, to more than an hour for more complex samples. Preliminary sample preparation may substantially increase the analysis time. Instrumentation for gas chromatography ranges in price from inexpensive (a few thousand dollars) to expensive (>,000). Gas chromatography. Gas chromatography is a widely used chromatographic technique that operates on the principle of “gas-liquid” chromatography. In this method, the stationary phase is a column, which is inserted into the instrument and contains a liquid stationary phase that is adsorbed onto the surface of an inert solid support. Headspace (HS) can be defined as the gas space above a sample when it is placed in a chromatography vial and therefore it is the analysis of the analytes present within that gas. Samples that can be analysed using HS analysis include anything that can fit in the vial and release volatile compounds, these include liquids and solids; here we will .
Gas chromatography (GC) at the time was more powerful than liquid chromatography (LC), . branched chain compounds can elute more rapidly than their corresponding linear isomers because their overall surface area is lower. . They are used in traditional quantitative analysis of samples and often use a UV-Vis absorbance detector. Narrow-bore .
A limitation in gas chromatography is that it can be used for only volatile materials Thus, non-polar materials are easy to separate than polar materials. However, ionic materials cannot pass through a gas chromatographic column. 6.2 PRINCIPLE OF GAS CHROMATOGRAPHY In gas chromatography, the compounds (mixtures) are separated by partitioning
Derivatization Reactions and Reagents for Gas Chromatography Analysis 85 ii. Analysis of relatively nonvolatile compounds. iii. Reduction of volatility of compounds prior to GC analysis. iv. Improvement of analytical efficiency and hence increase detectability. v. Stabilization of compounds for GC analysis. 2. Types of derivatization reactionsThe chromatography resolving power can substantially be increased by using multidimensional gas chromatography. This can be distinguished in two techniques, heart-cut and comprehensive. Using multidimensional chromatography can increase peak capacity (selectivity), increase sensitivity, reduce interferences and reduce analysis times. Gas Chromatography (GC) is a fundamental analytical technique that plays a pivotal role in modern scientific research and industry. With its remarkable ability to separate and quantify complex mixtures of volatile and semi-volatile compounds, GC has earned a prominent place in fields ranging from environmental analysis to pharmaceutical development. Ask the Chatbot a Question Ask the Chatbot a Question gas chromatography, in analytical chemistry, technique for separating chemical substances in which the sample is carried by a moving gas stream through a tube packed with a finely divided solid that may be coated with a film of a liquid.Because of its simplicity, sensitivity, and effectiveness in separating .
Image 1: The image above shows how a gas chromatograph looks like. Picture Source: hiq.linde-gas.com It is a term used to describe analytical separation methods used to check volatile substances in their gas phase. What happens during gas chromatography is that the components of a sample are dissolved in a solvent and vaporized to separate the analytes.
The silica gel (or the alumina) is the stationary phase. The stationary phase for thin layer chromatography also often contains a substance which fluoresces in UV light - for reasons you will see later. The mobile phase is a suitable liquid solvent or mixture of solvents. Gas chromatography (GC) separates and analyzes volatile compounds to determine sample purity through differential partitioning between a carrier gas and a stationary phase. GC is widely used in various fields like . Gas chromatography mass spectrometry (GC-MS) is an analytical technique that combines two powerful techniques; gas chromatography and mass spectrometry and is used to separate, identify, .
8.2 Gas Chromatography (GC) GC with flame-ionization detection (FID) is widely used for lipids analysis. Sample preparation for this technique includes preseparation of lipid classes, hydrolysis, derivatization, or pyrolysis. GC can also be used for direct separation of triacylglycerols based on the carbon number (CN).
Gas Chromatography 1 Gas Chromatography: Identifying Unknown Compounds Author: V. M. Pultz Last Update: October 22, 2015 Introduction Most materials that we encounter are mixtures, and this includes food, gasoline, car tires, sea water, animals, and the list goes on and on. Often we want to determine the composition of a Comparing the retention times or t R values of known compounds analyzed under the same conditions as the mixture . then it is termed as liquid chromatography (LC), and if it is gas, then it is called gas chromatography (GC). These two can be used for either preparative or analytical applications. . when using a mixed mode ligand containing .
Field grown plants of holy basil (Ocimum sanctum L.) were treated with biologically effective supplemental ultraviolet-B (sUV-B) radiation (ambient +1.8 kJ m−2 day−1) to evaluate its effect on specialized oil glands and quantity and quality of the essential oil. The total yield of essential oil increased significantly by 42.0% after sUV-B treatment. Gas .Chromatography separates compounds in a mixture from . UV light, the places where the compounds are on the plate will appear as dark spots on a . Figure 3.1 shows a TLC plate for a mixture that contains 3 compounds. The darkness 1. of the spot is a qualitative indicator of how much of a particular compound was in the sample. In the example .Gas chromatography (GC) is a powerful analytical tool, particularly in the petrochemical industry. . Petroleum samples are some of the most complex matrices: gasoline contains hundreds of compounds, and some of the higher-carbon cuts can contain thousands. Most detectors cannot provide any qualitative information of eluting analytes .
Basics & Fundamentals: Gas Chromatography. Introduction . Gas chromatography (GC) is an analytical methodology, which was devised by Nobel Laureate, Martin, et al. in 1952. More than 60 years after the award, GC systems are widely commercialized and used in various industries, capable of both of quantitation and qualifcation.
This lab involves the analysis of different unknown mixtures by high resolution capillary gas chromatography (GC) with an ion trap detector (ITD). . but the detector (the ITD) also provides information concerning the structure of each of the components. Therefore, compounds can be identified not only by comparing the retention time to a .
If analyzed under identical conditions, any given compound always produces the same family of ions, creating a unique mass spectrum for each compound. When two or more compounds are present, the mass spectrum is a combination of the spectrum of each component. The result may be so messy it can’t be used to identify the components, meaning MS .
The compound can be located by using physical and chemical methods. Physical methods. By radioactive method: If the substance is radiolabelled, it can be detected by counter devices. Fluorescence method: Some compounds which aren’t visible under ordinary light, fluorescence when held in ultraviolet (UV) light. Eg: fumaric acid, malic acid.
uv spectroscopy in gas
Matsuri Sushi Bar, Taubaté: Veja 2 dicas e avaliações imparc.
can uv containing compounds be analyzed under gas chromotography|gas chromatography uv spectroscopy